Understanding Fentalogs (Fentanyl Analogs)
- Marco Troiani
- Jan 27, 2025
- 10 min read
The opioid epidemic is claiming an accelerating number of lives in the United States every day. As the crisis continues, toxicology reports are showing that many opiate addicts are overdosing due to heroin and other opiates being laced with high-potency opioids such as fentanyl (1,4). More recently the phenomenon seems to have spread to pharmaceuticals, which are increasingly counterfeited by Mexican cartels with fentanyl as the active ingredient (1,3). These are replacing older-style powder drugs in the illicit market as a younger generation prefers diverted pharmaceutical pills. This move to a preference for pharmaceuticals is partially motivated by the fentanyl epidemic and the uncertainty it created in the illicit drug market, especially surrounding powder street drugs (1).

As US. authorities begin monitoring for fentanyl, illicit fentanyl manufacturers began switching to new analogs of the fentanyl molecule that were still psychoactive and therefore lethal, but evaded chemical detection by law enforcement (2,3,6). What this has created is a large number of fentanyl analogs, many of which cannot be detected by standard analysis at this time, that are being found in the drug supply and in the bodies of overdose victims (5,6).

Using the technique of retro-synthetic analysis, we can see the origin of illicit fentanyl and particularly its analogs, called fentalogs, from its chemical structure. For example, the diagram in Imageβ shows that with 4 starting reagents fentanyl can be synthesized: phenethyl bromine, 4-piperidone, aniline, and propionic acid. As the analogs we find in overdose patients and drug samples become more varied, we have more variations in reagents and starting materials to match to drug supplies whether overseas or domestic (3,5,6).
The remainder of this document is a technical focus on the organic chemistry of the analogs of fentanyl that are appearing. What this document does is present these analogs as variations upon the fentanyl molecule as a parent structure to identify both the proper nomenclature and molecular structure for each known analog. This is important because nomenclature has many consequences for law and regulation and proper molecular structure has many important consequences for laboratory analysis of a chemical compound.
The linkage between these two worlds of law and science is critical for the measures that are being proposed to stem the flow of fentanyl into the United States and to reduce fentanyl-related deaths. Particularly, the linkage between molecular structure and clandestine/criminal operations which are part of the process of enforcement of, and amendments to, the laws (3,5,6).
For a comprehensive list of known fentanyl analogs please see our related upcoming post Fentalog Detection Proposal.
For a list of relative pharmacological potency of fentanyl analogs where known, please see our related document Fentanyl Testing Sample Prep.
Fentanyl Analog Nomenclature/Structure Framework Using Retro-synthetic Analysis
Because there are so many analogs of fentanyl, the naming for them tends to follow classical or IUPAC nomenclature conventions. This section is written to help illustrate the basic ring structures of fentanyl, helping a chemist working with fentanyl analogs to quickly and consistently navigate the nomenclature system.
Note: Use the Digamma hyperlink in the bottom left of each page to return to the table of contents.
PART I: Organic Acid Substitutions
The synthesis of fentanyl analogs is done by reacting the ring structure as a base, 4-ANPP also called 4-anilino-N-phenethylpiperidine or despropionylfentanyl. 4-ANPP acts as a base because of its two amine nitrogens, the secondary amine acts as a base to react with an organic acid which condenses into an amide. Depending on the organic acid used in the amide condensation, different analogs of fentanyl will be produced. To help align this phenomenon in the eyes of chemists, we have labeled the precursor 4-ANPP with a [0.], and then the reactions synthesizing fentanyl, acetylfentanyl, butyrylfentanyl, and benzoylfentanyl with a [1.], [2.], [3.], and [4.] respectively. To further aid in the chemical pedagogy we have aligned each reaction number with a precursor -> product system that follows the following scheme:
EXAMPLE: reaction number -> precursor -> product [compound name]
1 -> α -> a [fentanyl]
2 -> β -> b [acetylfentanyl]
3 -> γ -> c [butyrylfentanyl]
4 -> δ -> d [benzoylfentanyl]
Using the above scheme, a chemist can quickly extrapolate the reactions necessary for other fentanyl analogs with more complex organic acids, such as cyclopropyl fentanyl or cyclopentyl fentanyl, or any other fentanyl analog derived from a reaction of 4-ANPP with any organic acid.

PART II: Hydrocarbon Substitutions

The modifications covered in this section have to do with carbon skeleton modifications of the original fentanyl molecular structure. These are organized into methyl formate additions, which are most known for the fentanyl -> carfentanil conversion. Many analogs of great potency, such as ohmfentanyl and lofentanil possess methyl formate groups added to the 4-carbon (of the piperidine ring). The methyl formate is added here from the carbonyl-carbon of the acetate moiety as it is with ohm- and lofentanil. The 4-carbon is not a chiral center in carfentanil because of a lack of piperidinyl substituents, but this same carbon is a chiral center in both ohm- and lofentanil because both of those analogs have asymmetrical piperidinyl substituents.
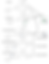
The second group is organized into methyl additions, which are known for the fentanyl analogs such as alpha-methylfentanyl and cis-3-methylfentanyl. These analogs can posses a wide variety of modified pharmacological properties, including increased and decreased potency (receptor binding efficiency), increased or decreased half-life (metabolic binding efficiency), or other effects on human physiology. Other substituents such as hydroxy, chloro, fluoro, and a wide variety of alkyl groups, are also substituted in place of these methylations to produce psychoactive analogs of fentanyl, but because they often use the same skeletal naming conventions as the simple methyl analogs, we did not reproduce them all in the image here.
For a more advanced challenge on nomenclature, consider the conflict of para-fluorofentanyl. With two phenyl groups, there are two structural interpretations of this name, a violation of IUPAC fundamental rules. Although existing literature conflicts on how to resolve this, this will likely be settled by guidance from IUPAC or a similar organization.

The way these conflicts are settled in organic chemical nomenclature is by assigning a primary and secondary ring system through a system of prioritization. The secondary system is assigned prime (‘) values to distinguish them from the substituent on the primary ring system. This is analogous to how the Greek letters on the organic acids are secondary to those on the phenethylamine chain shown in Image02 above.
PART III: Structure Modifications
The modifications described here cover alterations to the ring structure donated by the fentanyl precursor 4-ANPP. While the previous section covered substitutions of hydrogen atoms on the original carbon ring structure, sometimes called functional group additions, this series focuses on the three more complex modifications to the phenethyl moiety (i.e. the phenethylamine sub-structure).

The first modifications is the removal of the phenethyl moiety from the piperidinyl nitrogen, depicted here as hydrolysis yielding phenethanol. This changes the parent skeletal name to norfentanyl. This is analogous to how norepinephrine represents a demethylated epinephrine in human endocrinology, with the “nor” prefixing meaning “normal”. The second and third modifications are the removal and addition of a methylene moiety internally in the ethyl chain within the greater phenethyl moiety. The removal of a methylene, which shortens the chain by one carbon length, creates the benzylfentanyl structure. The addition of a methylene, which lengthens the chain by one length, creates homofentanyl. This is analogous to how homocysteine is an analog of the amino acid cysteine (Cys)[C] with a chain elongation by a methylene.
PART IV: Basic Stereochemistry
The modifications covered in this diagram have to do with stereochemistry and the assignment of unique Cahn-Ingold-Prelog R/S assignments to complex analogs of fentanyl. The stereochemistry of fentanyl analogs can seem at first counter-intuitive, because of the complex and unique nature of the reasoning organic chemists must develop to internalize complex three dimensional geometries such as those needed to comprehend stereochemistry. Thankfully these images follow a simple procedure for organizing a potential analog of fentanyl into the total number of unique stereoisomers, the number of true stereocenters on the molecule, and the number of Cahn-Ingold-Prelog R/S assignments that are appropriate for that analog.
The procedure used in the analysis of stereochemistry in these series of images is the following:
First the image is drawn out.
Second asterisks are placed by potential stereocenters as indicators (*).
Third the pairs of each stereocenters R and S orientations are combined through all possible permutations of stereocenters.
Fourth the permutations are checked for superimposability, indicating that they are varying around what is not a true stereocenter and therefore the possible permutations are truly the self-same stereoisomer.
This procedure will be used when making an assessment of the number of valid stereoisomers that an analog of fentanyl will be predicted to have. For those not as familiar with chemical chirality, this can seem like a very complex set of rules that at first seem paradoxical. But the mechanics follow the rules of organic chemistry and chirality and the parallel field of optics and photochemistry which have been used to collect data to validate these hypotheses about which molecules are a unique chemical phenomenon and which are merely the self-same molecule diagrammed in a separate method. A review of concepts such as electronic state and isoelectronic quantum systems is critical to see the equivalence or non-equivalence of molecular species. This equivalence or non-equivalence is the central task being performed when potential stereocenters are evaluated as true stereocenters. This process creates possible permutations of chiral configuration and determines that they are either unique molecular species or rather self-same molecules merely diagrammed differently. Use of plastic or digital 3D models for comparison of superimposability may be helpful to perform some of the procedural tests that are needed to determine the equivalence or non-equivalence of a given compound.
Because the analogs of fentanyl are so large, and the moieties (i.e. sub-regions) of the molecule that are relevant to its chirality are so small, redundant material consumes a lot of space on the page. We reproduce the fentanyl analog as a smaller, simpler molecule with the dynamics between its stereocenters. saving significant space.
The first case studied here is fentanyl itself, or propionyl-4-anilino-N-phenethylpiperidine, the most well known of the fentanyl analogs and the eponymous molecule (namesake) for the whole chemical category. The only identified potential stereocenter is found at the 4-carbon, opposite the piperidine nitrogen but adjacent to the aniline nitrogen. This seems to be a stereocenter because of the apparent four unique substituents (i.e. binding partners). Once we draw out the potential stereoisomers, we see that the two structures are superimposable in three dimensions, and therefore are the self-same molecule. For this reason fentanyl does not have R/S assignments, see Image06 for an illustration of these concepts.
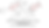
The second case studied here is of 3-methylfentanyl. There are two potential stereocenters, at the 4-carbon and also at the 3-carbon, where there is additionally a methyl group. Now, we mark both 3 and 4 carbon as potential stereocenters with an asterisk (*), and see how many potential stereoisomers we can eliminate. We see that of the four permutations of stereoisomers, none are superimposable in three dimensions, meaning each is a unique stereoisomer and that both potential stereocenters were true. This gives 4 potential R/S assignments, 4R3S, 4R3R, 4S3S, 4S3R.
What is particularly interesting and quite a contrast from the previous example of cyclohexanol, is the stereocenter at C-4 is indeed a true stereocenter here, whereas in the previous example of cyclohexanol as an analogy to fentanyl, the C-4 was not a true stereocenter. This changed because the modification of the C-3 carbon without an equivalent change on the C-5 carbon created an imbalance between two formerly identical substituents, creating a novel stereocenter where there was not one previously.

PART V: Advanced Stereochemistry
Advanced stereochemistry is no different than basic, but with many more elements per example and therefore further familiarity is recommended for fluency of subject matter. What happens in advanced models is that permutations can escalate to eight or more possible permutations can be considered for a single molecule. In addition, some of the the complexity will arise from the cases of more elements, the elimination of possibilities will also require some mastery to properly comprehend the subtle photochemistry and spatial geometry involved. If needed please review the concepts outlined at the top of Part IV: Basic Stereochemistry.
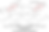
The third case studied here is alpha-methylfentanyl. This group contains a substitution similar to the 3-methylfentanyl that was examined in the previous example in Image08, the difference being the substitution is on the phenethyl chain as opposed to being on the piperidine ring. We then mark the two stereocenters, one on the C-4 carbon just as on fentanyl itself, and the other on the C-α (alpha carbon). When we draw out all potential stereoisomers, we see that the C-4 stereocenter is superimposable, eliminating it as a true stereocenter. This leaves only 2 R/S assignments that follow the orientation of the stereocenter at the C-α (alpha carbon) position. These are labeled αS and αR, a reflection of the stereocenter in the piperidine derivative being at C-α position. It is interesting that the C-4 stereocenter, which has no chiral activity in fentanyl (Image06), activates it in 3-methylfentanyl with a on-the-ring addition, and loses it again in alpha-methylfentanyl with an off-the-ring addition. This is because of symmetry breaking on the ring removes achirality / photochemical neutrality by creating substituents that are no longer isoelectronic to each other, from a quantum mechanical perspective.
The fourth case studied here is ohmfentanyl, which has 3 potential stereocenters. These are the 4-C, the 3-C, and the β-C (beta carbon). When we draw all potential stereoisomers out we see that they are not superimposable in any combination of pairing, and therefore we have 8 unique stereoisomers and 3 true stereocenters. This yields 8 unique R/S assignment combinations which are illustrated as captions under the stereoisomers, as shown in Image09.
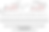
Notice the symmetry breaking discussed in the previous example of alpha-methylfentanyl (Image08) is occurring here with ohmfentanyl, creating a true stereocenter at C-4. This is because the four substituents bonded to C-4 are unique from a quantum mechanical perspective (i.e. none are isoelectronic).
These fundamentals are typically enough to help chemists navigate the world of fentanyl analogs proficiently. Other substituents and substitutions generally follow the naming conventions outlined in this section.
See the companion article on Linked-In here.
Citations
United States Drug Enforcement Administration “Fentanyl Flow to the United States” (2020) DEA Intelligence Report DEA-DCT-DIR-008-20 (DEA-PRB-01-08-20-01)
Varfaj, Ina, et al. "Original enantioseparation of illicit fentanyls with cellulose-based chiral stationary phases under polar-ionic conditions." Journal of Chromatography A 1643 (2021): 462088.
Roda, Gabriella, et al. "Ten years of fentanyl-like drugs: a technical-analytical review." Analytical Sciences 35.5 (2019): 479-491.
WHO Expert Committee on Drug Dependence, and World Health Organization. WHO Expert Committee on Drug Dependence [meeting held in Geneva from 9 to 16 April 1987]: twenty-fourth report. World Health Organization, 1988.
Guerrieri, Davide, et al. "Postmortem and toxicological findings in a series of furanylfentanyl-related deaths." Journal of Analytical Toxicology 41.3 (2017): 242-249.
Rue, Emily. Characterization of Fentanyl analogs by Instrumental Analysis. Diss. University of Illinois at Chicago, 2018.
